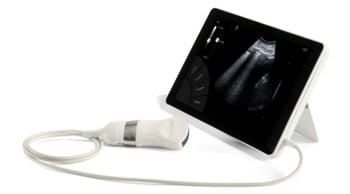
SONOSCANNER is announcing FDA (Food and Drug Administration) approval of the ultra-portable T-Lite Ultrasound for the American market
FDA Approval, guarantee of quality
This approval, obtained on 15th October, 2020, places Sonoscanner among the few French-technology companies to have satisfied the rigorous and demanding requirements of the American Public Health Authorities. It is also an additional confirmation of the quality of the Sonoscanner ultrasound devices, and represents a key milestone in the development of the Sonoscanner international growth strategies, by authorizing the commercialization of the T-Lite Ultrasound in the American market.
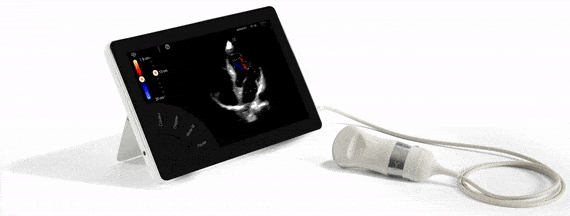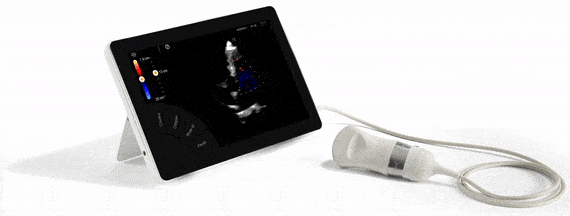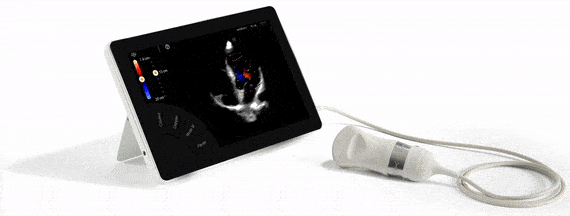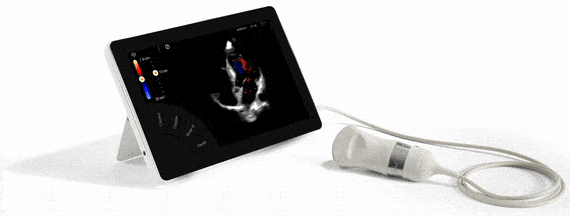
T-Lite, échographe ultra-portable, ultra-performant
The ultra-portable T-Lite model is the only ultrasound worldwide that combines an ultra-portability in tablet format, with an exceptional imaging quality, all the Doppler frequencies and a feature-rich functionality (private Cloud functions, automated reports).
“T-Lite is a very effective diagnostic tool,
ergonomic and easy to use”.
Doctor Eric Royer
General Practitioner and Sports Physician
A major contribution to the diagnosis of COVID-19
Furthermore, this particular Ultrasound model turns out to be highly useful in this period of COVID-19 pandemic, as it greatly facilitates the diagnosis and rapid dispatching of infected patients. Over and above the provided imaging quality, its compact size and weight (17 x 25cm, 1Kg) allow it to be easily moved from one patient bedside to another, thus lowering the risk of contamination. The tablet format makes disinfection particularly easy and quick (one minute as compared to close to one hour for a traditional ultrasound device).
The T-Lite Ultrasound is bound for strong growth in the American market.